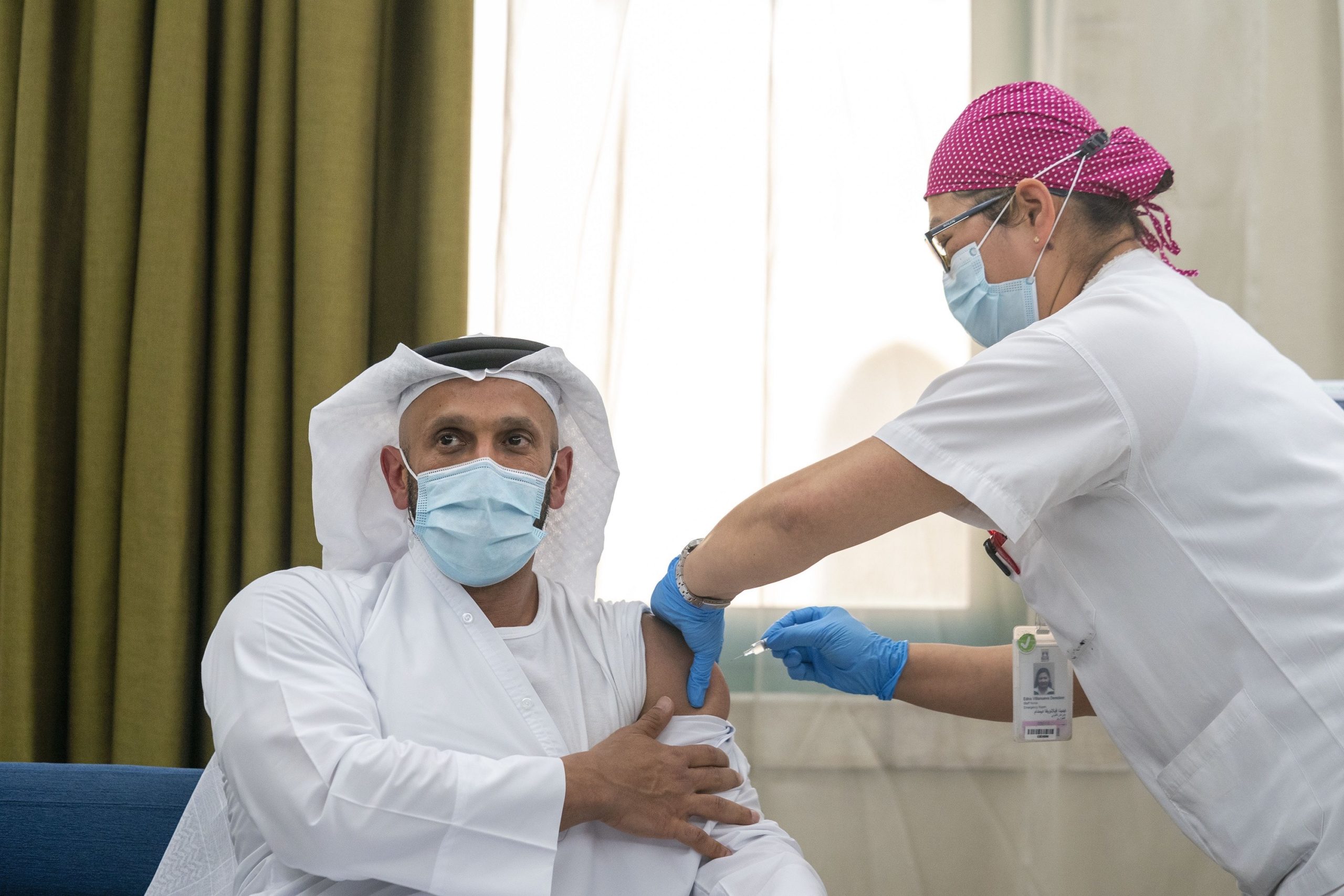
The first phase III clinical trial of a Covid-19 vaccine has begun in the UAE and is expected to involve up to 15,000 volunteers.
During the first two stages of the trial, which were conducted in China, 100 percent of volunteers generated antibodies after two doses in 28 days.
Sheikh Abdullah bin Mohammed Al Hamed, chairman of the Department of Health, Abu Dhabi, was the first person to participate in the World Health Organization-enlisted trial. The Department’s acting undersecretary, Dr Jamal Al Kaabi, was the second volunteer to trial the vaccine at Sheikh Khalifa Medical City on Thursday, July 16.
The trial is the result of a cooperation partnership between Abu Dhabi-based G42 Healthcare, which has been at the forefront of the battle against COVID-19 in the UAE, and, the world’s sixth largest vaccine manufacturer, Sinopharm CNBG.
Abu Dhabi Health Services (SEHA) officials are running the trial at five of their sites in Abu Dhabi and Al Ain, in addition to a mobile clinic, to ensure they are readily accessible to volunteers.
“Our participation in this trial enables us to make a major contribution in the global fight to combat the COVID-19 pandemic,” said Dr Nawal Ahmed Alkaabi, UAE Principle Investigator, Sheikh Khalifa Medical City chief marketing officer and chairperson of the National COVID-19 clinical management committee. “It is a matter of national pride that we are able to help facilitate the trial process that could have a worldwide impact and help people around the world to benefit from research and — if successful — the manufacture of a vaccine to fight back against this disease.”
The phase III trials will be open to individual volunteers aged between 18 and 60 living in Abu Dhabi and Al Ain and will last for three to six months, with the volunteers required to be available for follow ups during this time.
G42 Healthcare has ramped up efforts over the past few months that include establishing a throughput laboratory to speed up the detection of the disease; manufacturing essential PPE; conducting research into new vaccines and drug therapies and using its advanced artificial intelligence capabilities to map and predict trends in the outbreak, virus mutations and help combat the disease.
As home to more than 200 nationalities, the UAE provides robust research opportunities across multiple ethnicities — increasing the feasibility for a global application should the trials prove successful.
G42 and SEHA working towards gathering a minimum of 5,000 participants in the first stage of the trials, an effort that will be aided by a public awareness campaign to encourage UAE residents to participate.
The launch marks the start of a series of national initiatives aimed at enhancing the UAE’s medical research and development capabilities, including developing the local capacity to manufacture the vaccine.
The study, if successful, will be approved and accredited by the Ethics Committee for Scientific Research in the Emirate of Abu Dhabi. It is being supervised by the Department of Health Abu Dhabi and SEHA, following all international guidelines stipulated by WHO and the United States Food & Drug Authority.
• Volunteers interested in participating in the trials can now register on 4humanity.ae. A special hotline 02 819 1111 has also been created for all volunteers signing up for the trials.